Introduction - the disease and its effect on performance
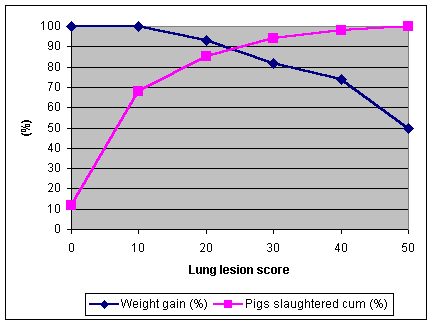
Burch (1982) related the effect of lung lesions (0-55) to the growth rate of pigs in the final four weeks before slaughter and also to the incidence in the pigs. As lung lesion scores increased the growth rate decreased, but it only affected 30% of the pigs with the higher lesion scores.
Graph 1. Effect of lung lesions scores on growth rate
Enzootic pneumonia, caused by Mycoplasma hyopneumoniae, affects over 90% of all pig herds and in severely affected farms over 90% of the pigs will have classical lung lesions at slaughter affecting the distal parts of the apical, cardiac and diaphragmatic lobes. On average approximately 40-50% of lungs show lesions at slaughter. The mycoplasma colonise the respiratory tract of the pig, gradually spreading down the bronchial tree, damaging the muco-ciliary escalator system, allowing the accumulation of material in the alveolar spaces, which cause the typical gross lesions. This enables the secondary bacteria to invade the respiratory tract, especially Pasteurella multocida and these can be isolated in over 40% of lung lesions. It has been shown that the mixed infection doubles the size of the lung lesion (Ciprian et al, 1986). Generally the mycoplasma infection alone is relatively mild and pulls down growth rate and feed conversion efficiency, but it is the secondary bacterial infections that exacerbate the disease and causes acute bronchopneumonia and even death.
Vaccination against M. hyopneumoniae has been shown to be effective in reducing lung lesions and improving growth rate, FCE and the general health of the pigs but does not totally eliminate the disease or the infection.
Graph 2. Effect of vaccination on lung lesions
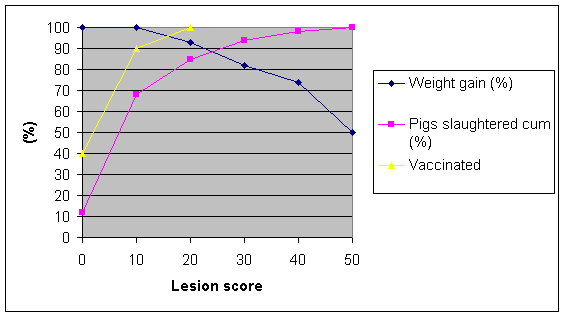
There has been some controversy over the extent of the percentage improvements in ADG but a recent paper by Baekbo et al (2002) looked at 8 Danish herds and examine 999 pigs at slaughter and 42% had enzootic pneumonia lesions. They had been monitored over a period of 7-24 weeks of age (Period 1) and over a shorter period 20-24 weeks just prior to slaughter (Period 2) when one would expect the pneumonia to be worse. Overall in Period 1 the growth rate was 698g/day and the pneumonic pigs grew 30.3g/day (4.3%) more slowly and in Period 2 they grew at 793g/day and the pneumonic pigs grew relatively more slowly by 58.5g/day (-7.4%). For every 1% of lung affected by EP lesions in Period 1 the growth rate was depressed by 2.8g/day (0.4%) and in Period 2 by 7.1g/day (0.9%). So if a herd had an average lung score of 10, which is quite a high over all score, then its growth would be reduced by 4% overall or 9% in the last month prior to slaughter.
Passive immunity - the effects of maternally derived antibodies and age on vaccinal response
A sow that is immune to M. hyopneumoniae or has been vaccinated herself, will pass on antibodies to her progeny via her colostrum. The colostrum contains very high levels of antibody, IgG being the dominant one (see graph 3). The piglet absorbs the antibodies mainly in the first 6 hours after colostrum consumption and intestinal closure to intact globulins occurs by 18 hours. (Bazer et al, 2001). Piglet serum levels are about the same as the sows by 24hours of age. Leukocytes found in the colostrum, such as macrophages, neutrophils and lymphocytes can also be absorbed whole in these early stages and they have been shown to have a protective effect as well.
Graph 3. Antibodies in the sow

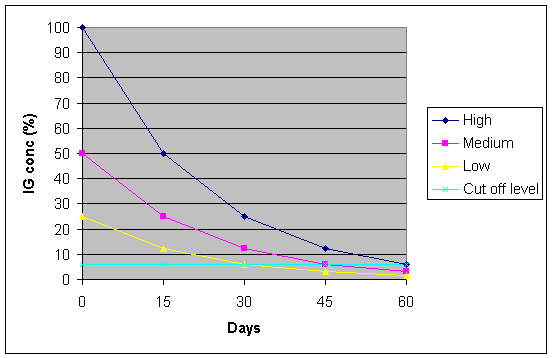
Once the piglet has absorbed the antibodies then the levels start to fall and the half-life for M. hyopneumoniae antibodies is 15.8 days (Ross, 1999). So a piglet with high initial antibody levels could still have significant levels 60 days later but one with only low levels could be clear by 30 days of age.
Graph 4. MDA depletion in piglets (example)
A recent report (Hodgins et al, 2002) looked at the effects of MDAs on vaccinal response in young piglets. Litters from 20 sows were used and the piglets were vaccinated at 2, 3, & 4 weeks of age. Blood was taken just prior to vaccination and 3 weeks after and serum antibody levels (IgG) were assayed using inactivated antigen from the mycoplasma vaccine culture.
Graph 5 Effect of MDAs on IgG response to vaccination
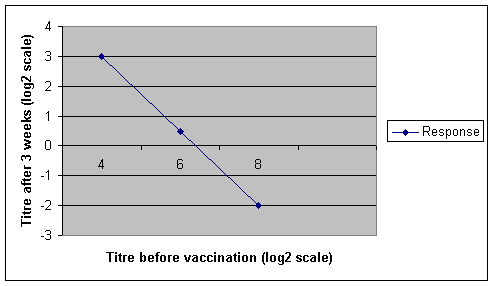
It was reported that vaccinated piglets had higher AB levels than unvaccinated. Higher pre-vaccination IgG titres gave significantly lower responses and a vaccine neutralization effect was observed at these high levels, i.e. the post-vaccination levels fell. Obviously this would have an adverse effect on the percentage vaccination response rate especially with a single shot vaccine. Age at vaccination (2, 3 & 4 weeks) had no significant effect on IgG response and there was minimal variation in regression lines. This finding may be surprising but reassuring.
Active Immunity - non-specific defences (Tortora and Grabowski, 1996)
The body has a number of non-specific defence mechanisms to keep bacteria out or to destroy them when they enter the body. They also then start the chain of events to stimulate specific active immunity both humoral and cell-mediated. The non-specific defences will be summarised in this section.
Table 1. Non-specific defences (italics highlight important factors for EP)
Physical barriers – skin, mucosal surfaces
Chemical protection – sebum, hyaluronic acid, lysozyme, gastric juices, mucus
Antimicrobial substances
- Transferrins (bind iron and stops bacteria growing)
- Interferons (produced by lymphocytes, macrophages & fibroblasts; Type I – (alpha & beta) antivirus, Type II (gamma) increase phagocytosis and natural killer cell (NKC) activity)
- Complement (over 22 recognised, found in blood plasma and plasma membranes; classical pathway C3+AB+AG from bacteria creates cascade of complement, some cause activation of inflammation (histamine release from mast cells), some opsonisation by coating bacteria and aiding phagocytosis and others cytolysis by attacking the cell membrane
Cellular components
- Natural killer cells (found in the spleen, lymph nodes, bone marrow and blood. They kill microbes and tumour cells but do not have antigen receptors so are non-specific. They attack cells that do not have the body’s own marker antigens (MHC – major histocompatibility complex antigens) and use perforins to kill cells)
- Phagocytes
§ Neutrophils (leave blood and form the first line of defence)
§ Macrophages (monocytes or wandering macrophages follow the neutrophils)
§ Fixed macrophages (these stand guard in tissues e.g. histiocytes in skin and sub-cutis, kupffer cells in the liver, macrophages in the spleen, lymph nodes and red bone marrow and alveolar macrophages in the lung)
The phagocytes are major component of the body's defences (reticulo-endothelial system) and the process of phagocytosis is also important. Chemotaxis attracts the phagocytes to the site where either the microbes are or cells are damaged, or complement is activated and inflammation is occurring. The phagocyte attaches to the microbe or foreign protein and ingests it via a phagosome. It is then digested and destroyed by the phagolysosome and the remnants excreted but also the antigenic peptides produced are then combined with a MHC II molecule (body's own marker substance) and can be presented to T cells in the lymph node to develop specific immunity. Phagocytosis and presentation of the foreign antigen is a very important process, which leads to the next stage of specific immunity both cell-mediated and humoral immunity. Macrophages also play a vital role in the production of cytokines, like the interleukins (IL-1, IL-2, IL-6, IL-10, IL-12 & IL-18), tumour necrosis factor (TNF-a), which also regulate the cellular response.
Inflammation
Inflammation plays an important role in activating the defensive reaction and eventual immune response. Redness, pain, heat, swelling and loss of function are the major cardinal signs of inflammation due to the physiological changes such as vasodilation, increased permeability of blood vessels, allowing phagocyte migration and eventual tissue repair. This is all under chemical control of histamine, kinins, prostaglandins, leukotrienes and complement. With EP though, the infection is not so reactive making the response relatively minor, unless there is further bacterial invasion, and may explain why it is such a chronic infection. Another important consideration is the response to a vaccine. The level of reaction at the site of vaccination and availability and quantity of antigen to stimulate the immune response will be dealt with later.
Specific immunity
The importance of this defence mechanism is its specificity i.e. reaction to a particular foreign antigen and it can distinguish between self and non-self antigens. It usually takes about 11 days to activate. Memory is the second key feature, that once primed, the system can initiate a rapid and vigorous response at the second encounter through the memory clone cells, often within 4 days.
Lymphocyte cells - humoral immunity (B lymphocytes and plasma cells)
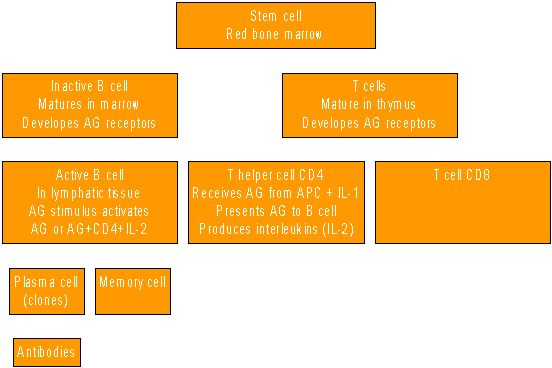
Lymphocytes form in the red bone marrow from stem cells. In the case of the B cells they mature there and are inactive but develop AG receptor sites. They become active B cells in lymphatic tissue and activated by an antigenic stimulus directly or by an activated T lymphocytic cell. The activated antigen stimulated cells become plasma cells, which multiply up as clones and secrete antibodies. Others become the memory cells ready for a second attack or vaccination.
Antibodies bind to antigens and inactivate them. IgM, which makes up about 5-10% of the immunoglobulin content in blood, is the first response antibody usually within 5-10 days of exposure. IgG is the major one with 60-75% in blood, appearing between 10-14 days and stimulates phagocytosis, neutralizes toxins and triggers the complement system. IgA makes up about 15% in blood is more important as a surface antigen, especially protecting mucous membranes and is key in EP as the organism is a surface organism mainly.
Figure 1. B lymphocyte development
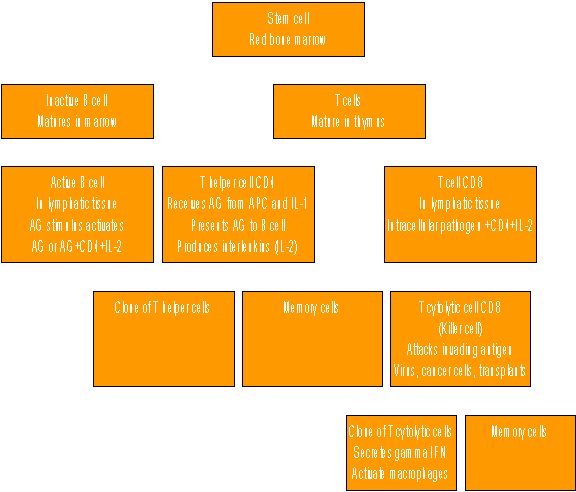
Lymphocyte cells - cell mediated immunity (T cells, helper CD4 & cytotoxic CD8) T cells are also produced from stem cells in the bone marrow but leave and mature in the thymus and develop their AG receptors and differentiate into different categories of cells with very different functions before going into the lymphoid system. The major cell types (clusters of differentiation) are CD4 helper cells and CD8 cytotoxic cells that attack cells that contain foreign antigens like virus or bacteria infected cells or tumour cells.
The helper cells are probably more important in EP. They receive antigen from the antigen presenting cells (APCs) such as macrophages and become activated and co-stimulated by interleukin 1 (IL-1) from the APC. The helper CD4 cell then activates and co-stimulates with IL-2 the B cell to go on and become a plasma cell and produce antibodies. This is also an important mechanism in EP and may in part explain the accumulation of lymphocytes and peri-vascular and bronchial cuffing that distinguish EP lesions. Both CD4 and CD8 cells proliferate and form activated clones and memory cells.
Figure 2. T lymphocyte development
Development of the pig's immune system
Table 2. Development of the foetal immune system of the pig
|
Day |
Development |
|
18 |
Stem & erythroid cells in the yolk sac |
|
28 |
Lymphoid cells found in thymus liver and blood |
|
40 |
T cells - clusters of differentiation (CD4 & CD8) in thymus, liver and blood |
|
55 |
Antigen-specific response detectable |
|
76 |
Interleukin-2 receptors on lymphocytes |
|
100 |
Natural killer cells in blood |
Parts of the system are developing in the foetus and are able to function as early as day 55. At birth the piglet is agammaglobulinaemic, immunologically naïve, because of the placental barrier.
Table 3. Development of the neonatal immune system of the pig
|
Cell type/function |
Neonatal |
Development |
Colostral |
|
Phagocytosis |
Low |
Develops over 12 wks |
|
|
Neutrophils |
High |
Decline to 3wks then increases |
Yes |
|
Macrophages |
Low |
Alveolar at 2 wks; intravascular 3-7 days |
|
|
Natural killer cells |
None |
See at 2-3 wks |
|
|
B cells |
Low 3-4% |
Mature 4 wks |
Yes |
|
T cells (CD4/CD8) |
Low 3-4% |
Mature 4wks |
Yes |
|
Memory cells (CD4+CD8) |
None |
Increase quickly to 6 months then steadily as adults |
|
|
Gut lymphoid tissue |
Poor |
Develops by 4 weeks |
|
Although the immune system is immature at birth, it is capable of responding immunologically. This response builds up quickly over the next 4 weeks and most are in place by 12 weeks of age. Importantly it does confirm that piglets as young as one week could respond to vaccination although the response will improve with age and improve the percentage response.
Protective effect of M. hyopneumoniae vaccines
Jayappa et al (2001) carried out studies in young piglets comparing vaccination at 1+3, 3+5 and 6+8 weeks of age with unvaccinated controls. Serology was carried out at the time of the first vaccination to determine the levels of MDAs against M. hyopneumoniae. The pigs were challenged at 16 weeks of age intratracheally, and necropsied at 21 weeks of age and the lung lesions recorded.
Graph 6. Protective effect of a vaccine given at different ages and different MDA levels
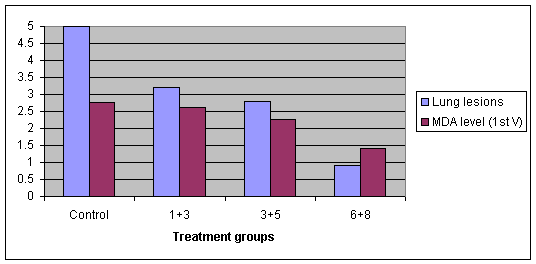
Lung lesions were reduced with vaccination even at 1+3 weeks of age. Vaccination at 3+5 weeks of age did not make much difference. As MDAs fell the vaccinal response improved. High MDAs do not prevent challenge later in life so really vaccination of the sow can be considered to have a negative impact on protection of the piglet through its productive life.
In a study by Thacker et al (2002) they also looked at the effect of age and MDAs on vaccinal response. Piglets were taken from vaccinated and non-vaccinated sows and they were vaccinated at 3, 6 & 9 weeks of age. Blood was taken at vaccination to assess MDA levels. They were challenged with M. hyopneumoniae at 14 weeks of age and necropsied at 18 weeks of age. As well as recording lung scores they carried out broncho-alveolar lavage to look at IgA and IgG levels there.
Graph 7. Protective effect of a single vaccination by age and MDA level
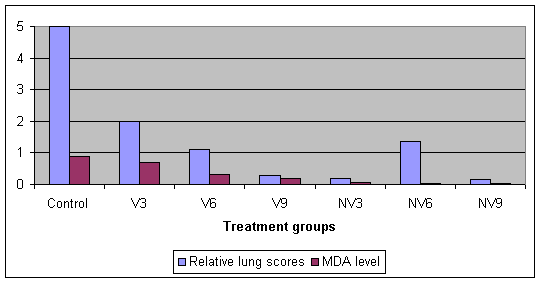
Graph 8. Protective effect of a single vaccination in comparison with IgA and IgG levels
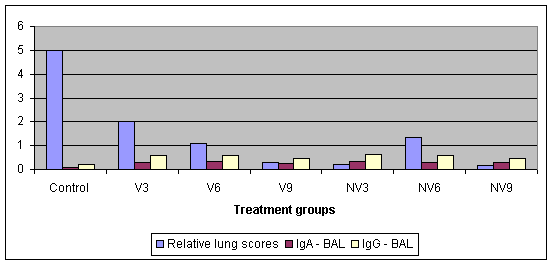
Overall there was a good trend that as MDA fell lung lesion fell, and comparing age from vaccinated and non-vaccinated sows there was little age impact but some group variation, which is common in EP studies. A single shot even as young as 3 weeks of age will give protection but it is not 100%. The stimulation of IgA and IgG do appear to be important, as all of the vaccinated groups had a much higher antibody level in comparison with the controls. This also highlights the poor immuno-response by the control pigs 4 weeks after infection, which is typical of EP and helps explains the chronicity of the disease i.e. the poor way the body fights it. MDAs can reduce vaccinal response so antigen content of a vaccine is a significant factor.
Adjuvants - effects on immunostimulation and protection
Trials were carried out looking at a range of adjuvants as part of a program to develop a new Actinobacillus pleuropneumoniae vaccine (Willson et al, 1995). Pigs were injected with different adjuvants to test and compare their irritancy over a 1-28 day period. Pigs were vaccinated twice with the test adjuvanted sub-unit vaccines at 6 and 9 weeks of age and challenged a week later. Antibody response and protective effect was measured, following the APP challenge and mortality and clinical scores were recorded.
Table 4. Effect of adjuvants on immunostimulation and protection
|
Adjuvant |
Irritation |
Antibody production |
Protection |
|
Corn oil |
+ |
+ |
++ |
|
Tocopherol (Vit E) |
+++ |
++ |
++ |
|
Mineral oil (Marcol) |
+(++) |
++++ |
++++ |
|
Aluminium hydroxide |
0 |
+ |
++ |
|
Oil in water emulsion |
(+) |
+++ |
++++ |
There has been much development over recent years in adjuvant technology. Some give a low reactivity at the injection site but a lower immune response e.g. corn oil and aluminium hydroxide. Some are very reactive such as mineral oil and tocopherol and the former can have a high viscosity and injection difficulties. Oil in water emulsions appear to growing in favour as they give optimum protection without being too reactive and have a low viscosity.
One M. hyopneumoniae vaccine with aluminium hydroxide adjuvant does appear to have a lower percentage performance (Martinon et al, 1998), by about 20% than other commercially available vaccines based on emulsions and may account for its poorer popularity. It gave approximately 50-70 % reduction in lung lesion in pigs from infected and non-infected farms in artificial infections and only 40-45% reduction in lesions in field trials. Vaccination at 1+4, 6+10 and 10 alone did not seem to make much difference to the response. Oil emulsion vaccines can give 70-90% lesion reductions in artificial challenge studies and approximately 60% in the field.
Graph 9. Trial results with an aluminium hydroxide adjuvanted mycoplasma vaccine

Antigenic load effect
There are many different products now appearing on the market, some recommend 2-shot and others 1-shot programmes. Generally for the 1-shot products the antigenic/mycoplasma content or load has been increased and they can still be injected into 3-4 week old piglets. Others recommend doubling the dose, which is convenient as you have the same pack, but can vary the program. Others recommend a single dose of the same two-dose vaccine but give it later at 10 weeks of age. Increasing the antigenic load/content is likely to improve the percentage response as it overcomes the risk from MDAs.
Table 5. Comparison of M. hyopneumoniae vaccines
|
Product |
2-shot |
1-shot |
Adjuvant |
Type |
|
Hyoresp |
2ml |
2ml |
ALOH |
Water |
|
Suvaxyn M. hyo |
2ml |
- |
Carbopol |
Polymer & water |
|
Mypravac suis |
2ml |
- |
Carbopol + Levamisole |
Polymer & water |
|
Stellamune (Once) |
2ml |
(2ml) |
Amphigen + Lecithin |
Oil in water |
|
Ingelvac M. hyo |
- |
2ml |
Impran |
Water in oil |
|
M+PAC |
1ml |
2ml |
ALOH + Emunade |
Oil in water |
Conclusions:
1. Maternally derived antibodies can play a major negative role in response to M. hyopneumoniae vaccines, by neutralizing vaccine antigen.
2. Age alone is not such a critical factor, although the immune system is immature at birth it is potentially active and has matured to a marked extent by four weeks of age. This applies to humoral and cell-mediated immunity.
3. Control of the infection is difficult to correlate directly with humoral or CMI activity and surface immunity (IgA) may prove more important.
4. M. hyopneumoniae vaccines are effective in reducing lung lesions but the response is variable.
5. Both 2-shot and 1-shot programs are effective. Neither prevents lesions developing by 100%, but do induce memory cells that fight challenge later.
6. Adjuvants are important to present the antigen in a more prolonged fashion and oil based ones appear to give improved percentage effect over aluminium hydroxide.
7. Antigenic load is important to achieve a good vaccinal response especially in single shot programs and if MDAs are present.
8. Which sow has a high level of circulating ABs, is an area of debate and future investigation and consequently, which offspring would benefit from a 1 or 2-shot program.
References:
Baekbo, P., Andreasen, M., Wachmann, H. and Christensen, G. (2002) Growth reduction in pigs with Pneumonia.
Proceedings of the International Pig Veterinary Society, Ames, Iowa, USA, 1, 283
Bazer, F. W., Ford, J. J. and Kensinger, R.S. (2001) Chapter 5. Reproductive Physiology. In Biology of the Domestic Pig, Editors Pond W. G. and Mersmann, H. J. Published by Cornell University Press, Ithaca, pp 150-224
Blecha, F. (2001) Chapter 16.Immunology
In Biology of the Domestic Pig, Editors Pond W. G. and Mersmann, H. J. Published by Cornell University Press, Ithaca, pp 688-711
Burch, D.G.S. (1882) The incidence and distribution of lung lesions, associated with enzootic pneumonia, in pigs from 2 farms, and the effect of the extent of these lesions on weight gains.
Proceedings of the International Pig Veterinary Society, Mexico City, Mexico, 95
Ciprian, A., Garza, M. and Pijoan, C. (1986) Interaction between Mycoplasma hyopneumoniae and Pasteurella multocida in conventional pigs.
Proceedings of the International Pig Veterinary Society, Barcelona, Spain, 282
Hodgins, D.C., Shewen, P.E. and Dewey, C.E. (2002) Influence of age and maternal antibodies on antibody responses of neonatal piglets to Mycoplasma hyopneumoniae.
Proceedings of the International Pig Veterinary Society, Ames, Iowa, USA, 1, 255
Jayappa, H., Davis, R., Rapp-Gabrielson, V., Wasmoen, T., Thacker, E. and Thacker, B. (2001) Evaluation of the efficacy of Mycoplasma hyopneumoniae bacterin following immunization of young pigs in the presence of varying levels of maternal antibodies.
American Association of Swine Veterinarians Conference, Nashville, Tennessee, USA, 237-241
Martinon, O., Tiberghien, M.P., Reynard, G. Blanchet, M., Brun, A. and Milward, F. (1998) Duration of immunity of a Mycoplasma hyopneumoniae bacterin (Hyoresp)
Proceedings of the International Pig Veterinary Society, Birmingham, UK, 3, 285
Ross, R.F. (1999) Chapter 36. Mycoplasmal Diseases.
In Diseases of Swine 8th edition, Edited by Straw, B. E., D'Allaire, S., Mengeling, W.E. and Taylor, D.J. Published by Iowa State Press, Ames, Iowa, USA, pp 495-509
Thacker, B., Wegner, M., Erlandson, K., Maxwell, K., Thompson, J. and Thacker, E. (2002) Influence of maternal antibody on mycoplasma vaccine Respisure One efficacy.
Proceedings of the International Pig Veterinary Society, Ames, Iowa, USA, 2, 307
Tortora, G.J. and Grabowski, S.R. (1996) Chapter 22. The Lymphatic System, Non specific Resistance to Disease and Immunity.
In Principles of Anatomy and Physiology 8th edition. Published by Harper Collins, Menlo Park, California, USA, pp 670-706
Willson, P.J., Rossi-Campos, A. and Potter, A.A. (1995) Tissue reaction and immunity in swine immunized with Actinobacillus pleuropneumoniae vaccines.
Canadian Journal of Veterinary Research, 59, 299-305
 Octagon technical papers on-line: index
Octagon technical papers on-line: index